Unit Operations 1 – Fluid Mechanics, Filtration, Heat Transfer, Evaporation, and Reaction Kinetics
FLUID MECHANICS, FILTRATION, HEAT TRANSFER, EVAPORATION, AND REACTION KINETICS
Examination Summary
0 of 40 Questions completed
Questions:
Information
You have already completed the examination before. Hence you can not start it again.
Examination is loading…
You must sign in or sign up to start the examination.
You must first complete the following:
Results
Results
0 of 40 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- Current
- Review / Skip
- Answered
- Correct
- Incorrect
-
Question 1 of 40
1. Question
The gas phase reaction 2A + B → R starts with 40% A, 30% B and 30% inerts. Reaction proceeds in a CSTR with CAo = 1.0 mole/liter. If the final concentration of A is 0.1 mole/liter, the % conversion will be
CorrectIncorrect -
Question 2 of 40
2. Question
Calculate the theoretical horsepower required to compress 100 cfm of carbon monoxide at -60°F and 1 atm to 10 atm in a single-stage adiabatic compressor. Use Cp/Cv = 1.3.
CorrectIncorrect -
Question 3 of 40
3. Question
Water at 21°C is being pumped at the rate of 40 m3/hr from a well-insulated storage tank to a second vented storage tank 25 m above the first tank. The motor driving the pump supplies energy at the rate of 8.5 kW. From the pump, water passes through a heat exchanger before it goes to the second tank. If the temperature of the water at the second tank is 26°C, determine the net heat added to the water in kW.
CorrectIncorrect -
Question 4 of 40
4. Question
Water leaves the 25 mm diameter nozzle of a fire hose at a velocity of 25 m/s. What will be the reaction force at the nozzle which the fireman will have to counterbalance?
CorrectIncorrect -
Question 5 of 40
5. Question
The temperature of the earth’s atmosphere drops about 5°C for every 1 km of elevation above the earth’s surface. If the air temperature at ground level is 15°C and the pressure is 700 mmHg, at what elevation is the pressure 380 mmHg. Assume air behaves as an ideal gas and neglect variations in gravitational force.
CorrectIncorrect -
Question 6 of 40
6. Question
A Boeing 747 jet is cruising in still air at 5°C and 100 kPa at a Mach number of 0.82. The velocity of the jet plane is
CorrectIncorrect -
Question 7 of 40
7. Question
The friction factor in turbulent flow in smooth pipes depends upon
CorrectIncorrect -
Question 8 of 40
8. Question
Which of the following is true?
CorrectIncorrect -
Question 9 of 40
9. Question
In the filtration of a sludge, the initial period is effected at a constant rate with the feed pump at full capacity until the pressure differential reaches 400 kPa. The pressure is then maintained at this value for a remainder of the filtration. The constant rate operation requires 900 seconds and 1/3 of the total filtrate is obtained during this period. No washing of the cake is done. If the time for removing the cake and reassembling the press is 1200 seconds determine the total cycle time that will give maximum capacity.
CorrectIncorrect -
Question 10 of 40
10. Question
A rotary vacuum filter with 30 % submergence is used to filter a slurry at a pressure drop of 1415 psf. The filter cycle time is 300 seconds. Determine the total area (in ft2) required to produce 1.41 ft3 of filtrate per cycle and 2.7 pounds of dry cake. Other data are:
α o = 2.90 x 1010 ft/lb
compressibility coefficient, s = 0.26
Rm = 0
T = 20°C, viscosity of filtrate = 1 cp ,and density of filtrate = 62.3 lbm/ ft3.CorrectIncorrect -
Question 11 of 40
11. Question
In order to maintain the rate of filtration constant, it is necessary that
CorrectIncorrect -
Question 12 of 40
12. Question
A Plate and Frame filter press with 1-inch thick frames operates at 25 psi with a filtration time of 7 hours. The cake is washed with water using one-half of the volume of filtrate collected. Dumping the cake, cleaning and reassembling the press takes 20 min. If the 1-inch frames were replaced with 0.75 inch frames, what will be the total cycle time for the 0.75 inch frames?
CorrectIncorrect -
Question 13 of 40
13. Question
A triple effect evaporator is supplied with 10,000 lb/hr of saturated steam at 240°F to the first effect. The temperature at the last effect is 150°F. Assume U1 = 150, U2 = 200, and U3 = 250 Btu/hr-ft2-°F, and the boiling point rises are 10, 18 and 27°F, respectively. Estimate the area needed for the first effect in ft2.
CorrectIncorrect -
Question 14 of 40
14. Question
A filter press was operated under constant rate for 20 minutes where 5 m3 of filtrate was collected. If the CRF operation is followed by CPF operation, how many more minutes will it take to collect another 5 m3 of filtrate?
CorrectIncorrect -
Question 15 of 40
15. Question
A current of 250 Amperes is passing through a stainless-steel wire (k= 22.5 SI units) with diameter of 5.08 mm. The wire is 2.44 m long and has an electrical resistance of 0843 ohms. The outer surface temperature is held constant at 427.6 K. What is the center temperature in K?
CorrectIncorrect -
Question 16 of 40
16. Question
When the flow of the fluid is laminar, the pressure drop across the length of pipe of known diameter may be best calculated using the
CorrectIncorrect -
Question 17 of 40
17. Question
A slurry of CaCO3 in water is filtered in a P and F filter press with a total area of 86.1 ft2, and operated at constant pressure drop of 2 atm. The mass of dry cake collected is 742 lbs and the volume collected is 68.1 ft3. The following data have been determined for the said slurry:
Viscosity = 0.982 cp; specific cake resistance= 1.27 x 1011 ft/lb;
resistance due to the filter medium= 1.55 x 1010/ft.
Calculate the filtration time in minutes.CorrectIncorrect -
Question 18 of 40
18. Question
A spherical furnace has an inside radius of 3 ft and an outside radius of 4 ft. The inside surface temperature of the furnace is 2000°F and the outside surface temperature is 175°F. The thermal conductivity of the wall is 0.201 Btu/hr-ft-°F. Estimate the temperature at a radius of 3.5 ft.
CorrectIncorrect -
Question 19 of 40
19. Question
Determine the net heat transfer by radiation between two flat parallel squares A and B 2 m x 2 m, one exactly above the other, 2 m apart. A is at 300°C with emissivity of 0.9 and B is at 100°C with emissivity of 0.25.
CorrectIncorrect -
Question 20 of 40
20. Question
A 2-inch outside diameter steam pipe is carrying steam at a temperature of 350°F. The pipe is covered with a coating material 2” thick ( k = 0.61 Btu/hr-ft-°F). If the outside surface of the coating is at 100°F, how much heat is lost per foot length of pipe?
CorrectIncorrect -
Question 21 of 40
21. Question
The black flat roof of a building has an emissivity of 0.9 and absorptivity of 0.8 for solar radiation. The sun beats down at midday with an intensity of 300 Btu/ft2-hr. If the temperature of the air and of the surroundings is 80°F, if the wind velocity is negligible, and if no heat penetrates the roof, what is the equilibrium temperature of the roof? The rate of heat transfer by conduction and convection may be estimated from q/A =0.38(delta T)1.25, where (delta T) is the temperature drop between the roof and air in °F
CorrectIncorrect -
Question 22 of 40
22. Question
A plane wall is composed of an 8-inch layer of refractory brick ( k = 0.75, English units ) and a 2-inch layer of insulation with k = 0.02 + 0.0001 T, where T is in °F. The inside surface temperature of the brick is 2000°F and the outside surface of the insulation is at 100°F. Calculate the temperature at the boundary between the brick and insulation in °F.
CorrectIncorrect -
Question 23 of 40
23. Question
A very long 1-cm diameter cylindrical rod (k = 300 W/m-K) has a base temperature of 250°C If it is exposed to ambient air at 28°C (h = 10 W/m-K), what will be the heat lost by the fin to the air by convection in W/m?
CorrectIncorrect -
Question 24 of 40
24. Question
The center-to-center distance of a bank of 2 in. O.D. tubes in a furnace is 5 inches. The average temperature of the tubes is 300°F while the furnace wall temperature is 1000°F What is the total heat transferred by radiation in Btu/hr-ft of tube that is directly intercepted by the first two rows of tubes, assuming tube and wall surfaces are black bodies?
CorrectIncorrect -
Question 25 of 40
25. Question
Heat transfer in turbulent flow may be described by an empirical equation correlating
CorrectIncorrect -
Question 26 of 40
26. Question
For an insulated steam pipe, say 1 inch diameter or higher, to save on heat losses, under normal operating conditions, it is best if the thickness of insulation will result in a radius that is _______ compared to the critical radius of insulation.
CorrectIncorrect -
Question 27 of 40
27. Question
What would happen if the kinetic energy of the reactants was not enough to provide the needed activation energy:
CorrectIncorrect -
Question 28 of 40
28. Question
A single-effect evaporator is used to concentrate 7 kg/s of solution from 10 % to 50 % of solids. The feed enters the evaporator at 294 K and its specific heat is 3.76 kJ/kg-K. Saturated steam is available at 205 kN/m2 and evaporation takes place at 13.5 kN/m2. The specific heat of the thick liquor is 3.14 kJ/kg-K. The overall heat transfer coefficient is 3 kW/ m2-K. The condensate leaves the heating space at 352.7 K. Estimate the heating surface required in m2 .
CorrectIncorrect -
Question 29 of 40
29. Question
Methane gas is being pumped through a 305 m length of 52.5 mm i.d. smooth steel pipe at the rate of 41 kg/( m2-s). If the outlet pressure is to be 298.4 kPa absolute, what pressure must be maintained at the inlet in kPa abs? Assume constant temperature of 288.8 K.
CorrectIncorrect -
Question 30 of 40
30. Question
A 2% caustic soda solution at 65°F is concentrated in a horizontal tube evaporator. The thick liquor leaves the bottom of the evaporator at 230°F and contains 30 lb of caustic soda per 100 lb of water. The pressure in the evaporator is 1 atm abs. Steam is available at 270°F If U = 300 Btu/hr-ft2-°R and F = 1000 lb/hr, Determine the steam economy.
CorrectIncorrect -
Question 31 of 40
31. Question
Oil flowing at 5.04 kg/s is cooled in a 1-2 shell and tube heat exchanger from 366.5 K to 344.3 K by 2.02 kg/s of water entering the shell at 283.2 K. Assume Uo = 340 W/(m2-K) and the specific heat of the oil is 2.09 J/g-K. Estimate the total outside surface area of the tubes required in m2.
CorrectIncorrect -
Question 32 of 40
32. Question
The boiling point rise of a 22% calcium chloride at solution temperature of 270°F is
CorrectIncorrect -
Question 33 of 40
33. Question
A liquid phase reaction
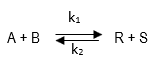
where k1 = 7 L/mol-min and k2 = 3 L/mol-min is to take place in a 120-L CSTR. Two feed streams, one containing 2.8 mols A / L, and the other containing 1.6 mols B / L, are to be introduced in equal volumes into the reactor, and 75% conversion of the limiting component is desired. What should be the flow rate of each stream? Assume a constant density throughout.
CorrectIncorrect -
Question 34 of 40
34. Question
A first-order liquid phase reaction was carried out in a batch reactor. After 15 minutes, 50% of the reactant was converted to the desired product. How many per cent would remain after 30 minutes?
CorrectIncorrect -
Question 35 of 40
35. Question
The reaction A → 2B is carried out in a batch reactor with CAo = 175 mols/li and CBo = 0. At T = 70°F, CB = 0.022 mols/li after 10 minutes, and at T = 100°F, CB = 0.059 mols/li after 10 minutes. What is the activation energy for this reaction?
CorrectIncorrect -
Question 36 of 40
36. Question
The reaction
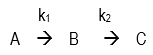
is carried out in a batch reactor. The initial concentrations of B and C are zero and that of A is 1 mol/L. k1 = k2 = k = 0.1/min. Determine the concentration of C at the time when the concentration of B is maximum.
CorrectIncorrect -
Question 37 of 40
37. Question
A second-order liquid phase reaction where CAo = CBo = 1 mol/L is carried out in a single CSTR where conversion is found to be 50 %. The rate constant is 0.05 li/(mol-s). If a PFR of 2-liter volume were hooked up in parallel with the existing CSTR, and assuming that 50 % conversion is also attained in the PFR, determine the total flow rates ( in L/min) processed by the two reactors.
CorrectIncorrect -
Question 38 of 40
38. Question
Aspartic acid is produced from ammonium fumarate (A) using aspartase immobilized in gel beads. The initial concentration of ammonium fumarate is 100 g/li. It is desired to convert 90% of the fumarate to aspartic acid. The rate reaction of the fumarate may be represented by
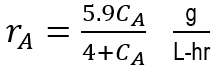
where: CA= concentration of the fumarate in g/liter. It is desired to treat 3 metric tons per month (30 days per month) of ammonium fumarate. If a PFR is used, what volume in liters is required? Assume constant volume conditions.
CorrectIncorrect -
Question 39 of 40
39. Question
Water flows through a 3-inch SCH 40 pipe at 60°F. A pitot tube is inserted at the center line of the pipe and registers a 3-inch Hg differential. What is the mass flow rate of water in lbm/s?
CorrectIncorrect -
Question 40 of 40
40. Question
For the liquid-phase batch consecutive reaction A —k1—–> B- –k2—-> C , k1 = 0.35/hr, k2 = 0.13/hr, CAo = 4 lbmols/ft3 and CBo =0, CCo = 0. What is the maximum concentration of B ( in lb mols/ft3 ) if the reactor used is a single CSTR?
CorrectIncorrect
